1. Introduction
Quality Control Materials (QCM) refer to samples used to control measurement quality, which meet the relevant requirements of ISO Guide 34 and 35 and are one or more samples with uniform specific parameters. Quality control samples are an effective extension of capability validation samples, providing important technical support for laboratory quality control.
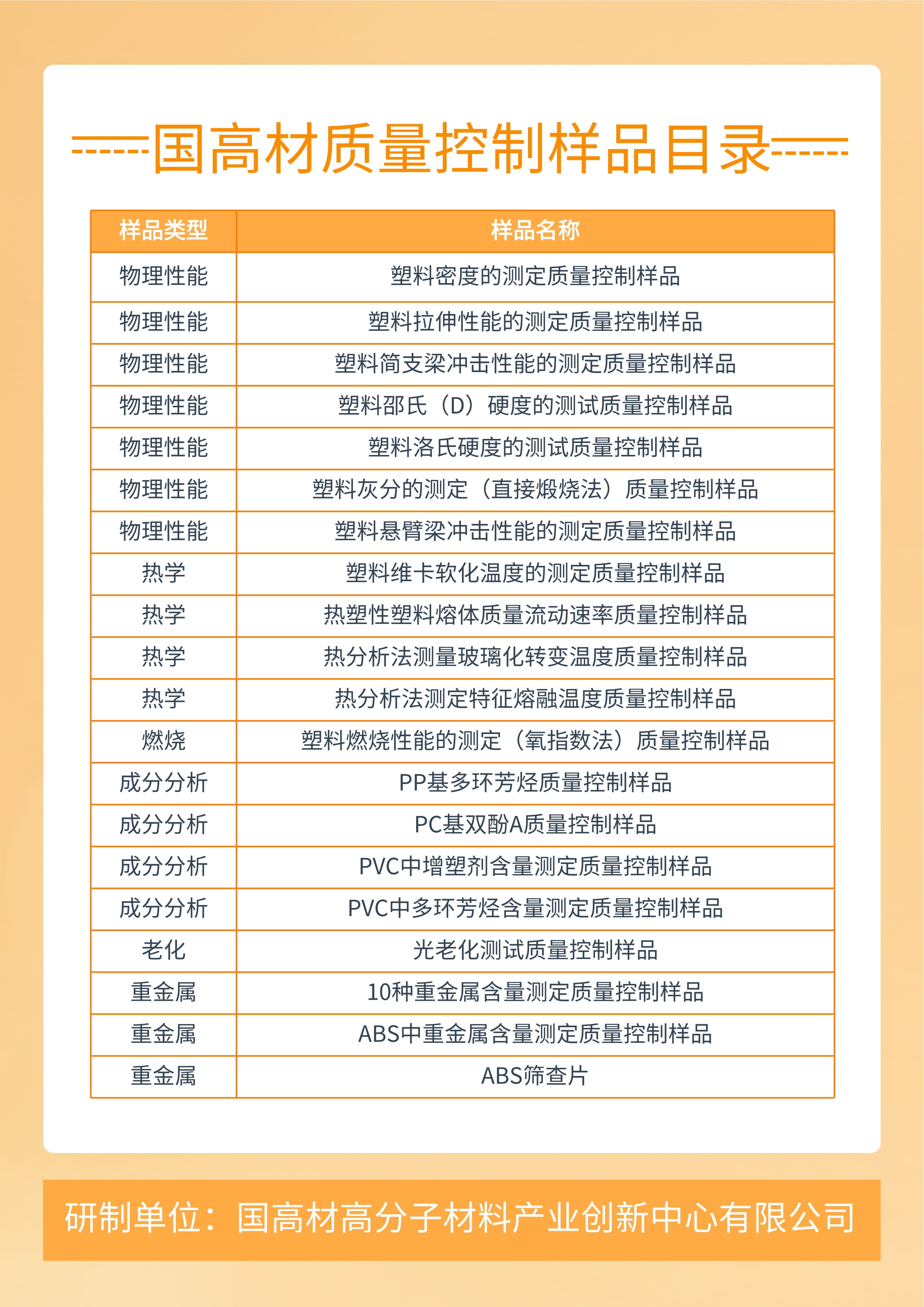
Based on a strong modified plastic platform and the rich experience of national engineering laboratories and academician workstations, as well as advanced complete sets of preparation equipment such as twin-screw extruders, torque rheometers, and injection molding machines, we have developed a series of stable and uniform laboratory quality control samples (QCM) in accordance with ISO/IEC 17043-2010 "General Requirements for Qualification Evaluation Capability Verification", which can prepare non-metallic material chemical properties Physical properties and other standard substances.
2. Characteristics
The variety of quality control samples (QCM) for national high-quality materials is abundant, and they meet the relevant requirements of ISO guidelines 34 and 35. They are close to the daily testing samples in the laboratory and meet the many needs of actual laboratory work. Its main characteristics include:
1) Developed by capability verification experts and senior material research and development engineers in accordance with national standards;
2) Meet laboratory testing requirements through meticulous processing;
3) Determine the accurate value through inter laboratory project comparison;
4) Provide detailed and clear sample usage instructions;
5) Develop a variety of plastic based samples based on market demand;
6) Exquisite and environmentally friendly packaging;
7) Reasonable pricing.
3. Sample Usage
1) Internal quality control in the laboratory;
2) New methods for laboratory evaluation and validation;
3) Verification of laboratory instruments and equipment during the period;
4) Participate in proficiency testing exercises in the laboratory;
5) Preparation of laboratory QC diagrams;
6) Consistency check of laboratory instruments;
7) Assessment and evaluation of technical abilities of analysis and testing personnel;
8) Various expected uses in ISO Guide 33.